
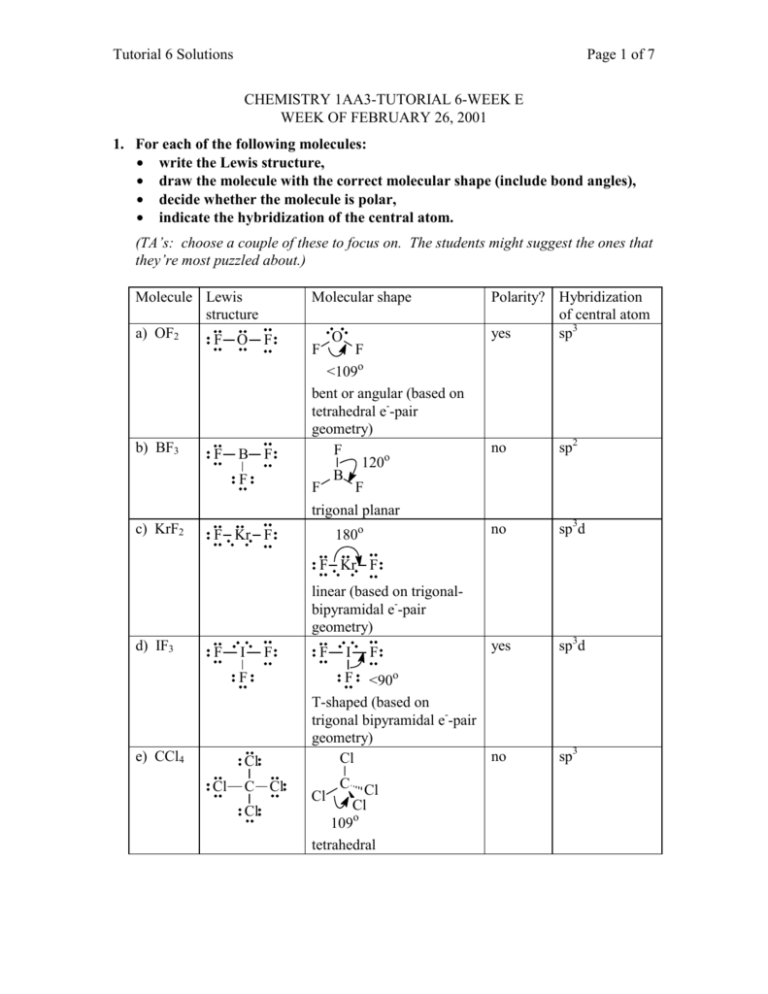
Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons. Octahedral: Six electron groups involved resulting in sp3d2 hybridization, the angle between the orbitals is 90°.
HYBRIDATION SP3D FULL
1 $\begingroup$ What is the difference between $\mathrm$ hybridization? The atomic orbitals of the same energy level can only take part in hybridization and both full filled and half-filled orbitals can also take part in this process, provided they have equal energy. Are they one in the same? School Walla Walla University Course Title CHEM 142 Type. (b) These orbitals combine to form a trigonal bipyramidal structure with each large lobe of the hybrid orbital pointing at a vertex. The repulsion between these groups produce a linear shape for the molecule with bond angle of 180. Sp and sp2 hybridization results in two and one unhybridized p orbitals respectively whereas in sp3 hybridization there are no unhybridized p orbitals. We're sorry, but in order to log in and use all the features of this website, you will need to enable JavaScript in your browser. * Each of these sp3 hybrid orbitals f… Generally, the Lewis structure is helpful to understand the molecular geometry of any given chemical compound. This intermixing is based on quantum mechanics. These hybridizations are only possible for atoms that have d orbitals in their valence subshells (that is, not those in the first or second period).
As there are molecules of Iodine, one molecule of Iodinewill be in the centre. Sp 3 d Hybridization sp 3 d hybridization is shown in phosphorus penta chloride (PCl 5 ).


 0 kommentar(er)
0 kommentar(er)
